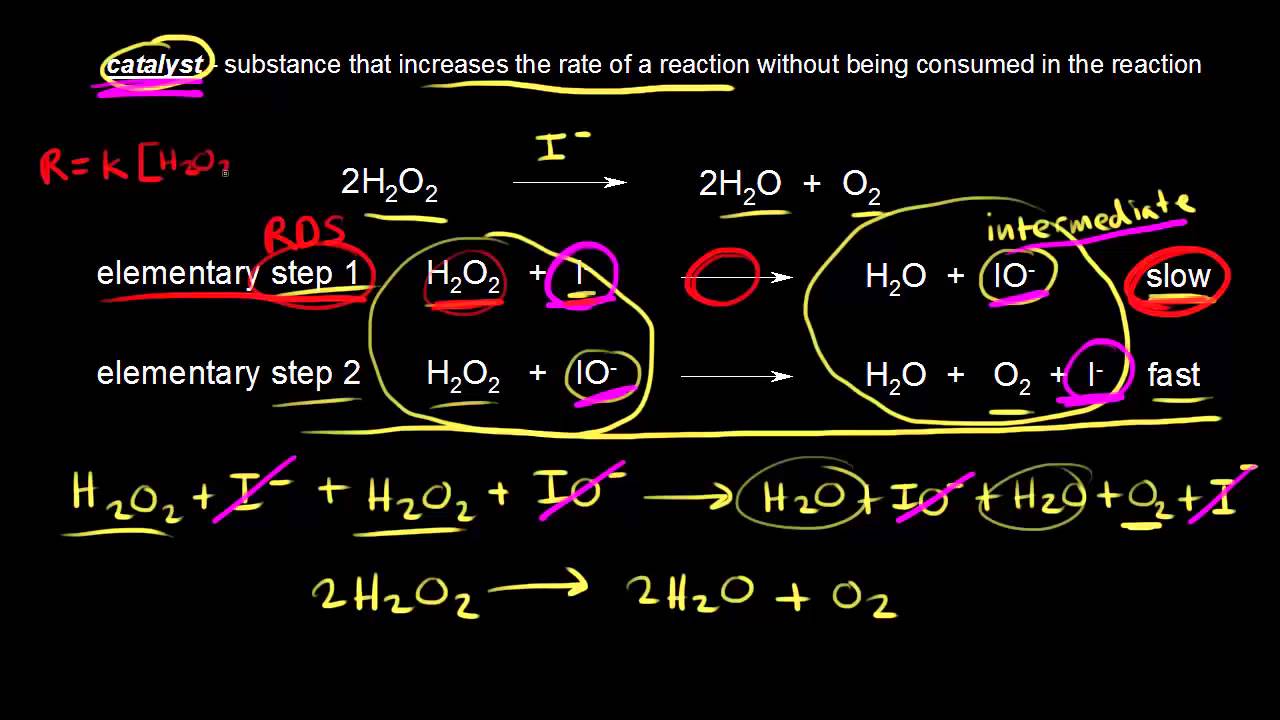
Catalyst In This Reaction . We have two different catalysts that speed up the. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. In heterogeneous catalysis, catalysts provide a surface to which reactants bind in a process of adsorption. what are catalysts, and how do they work in terms altering the parameters of a reaction? catalysts in biological reactions are called enzymes close enzyme a protein which catalyses or speeds up a chemical. catalysis is a process of increasing the rate of a chemical reaction by adding a chemical substance which is. a catalyst is a substance that speeds up the rate of a chemical reaction. catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Catalysts play an important part in many chemical processes.
from www.youtube.com
catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. In heterogeneous catalysis, catalysts provide a surface to which reactants bind in a process of adsorption. what are catalysts, and how do they work in terms altering the parameters of a reaction? catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. catalysts in biological reactions are called enzymes close enzyme a protein which catalyses or speeds up a chemical. Catalysts play an important part in many chemical processes. a catalyst is a substance that speeds up the rate of a chemical reaction. We have two different catalysts that speed up the. catalysis is a process of increasing the rate of a chemical reaction by adding a chemical substance which is.
Catalysts Chemistry Khan Academy YouTube
Catalyst In This Reaction catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. a catalyst is a substance that speeds up the rate of a chemical reaction. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. catalysis is a process of increasing the rate of a chemical reaction by adding a chemical substance which is. Catalysts play an important part in many chemical processes. catalysts in biological reactions are called enzymes close enzyme a protein which catalyses or speeds up a chemical. In heterogeneous catalysis, catalysts provide a surface to which reactants bind in a process of adsorption. We have two different catalysts that speed up the. what are catalysts, and how do they work in terms altering the parameters of a reaction?
From www.youtube.com
Catalysts Chemistry Khan Academy YouTube Catalyst In This Reaction catalysis is a process of increasing the rate of a chemical reaction by adding a chemical substance which is. Catalysts play an important part in many chemical processes. In heterogeneous catalysis, catalysts provide a surface to which reactants bind in a process of adsorption. a catalyst is a substance that speeds up the rate of a chemical reaction.. Catalyst In This Reaction.
From www.researchgate.net
Catalytic processes on a solid catalyst. Download Scientific Diagram Catalyst In This Reaction catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Catalysts play an important part in many chemical processes. We have two different catalysts that speed up the. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. catalysis is a process. Catalyst In This Reaction.
From www.researchgate.net
1 Schematic illustration of a catalytic process showing "A" and "B Catalyst In This Reaction In heterogeneous catalysis, catalysts provide a surface to which reactants bind in a process of adsorption. catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Catalysts play an important part in many chemical processes. what are catalysts, and how do they work in terms altering the parameters of. Catalyst In This Reaction.
From www.youtube.com
Identifying catalysts in a reaction YouTube Catalyst In This Reaction catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. catalysis is a process of increasing the rate of a chemical reaction by adding a chemical substance which is. what are. Catalyst In This Reaction.
From www.chemengonline.com
Catalysis Fundamentals Chemical Engineering Page 1 Catalyst In This Reaction In heterogeneous catalysis, catalysts provide a surface to which reactants bind in a process of adsorption. We have two different catalysts that speed up the. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the. Catalyst In This Reaction.
From www.sciencelearn.org.nz
Chemical reactions and catalysts — Science Learning Hub Catalyst In This Reaction catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. In heterogeneous catalysis, catalysts provide a surface to which reactants bind in a process of adsorption. a catalyst is a substance that. Catalyst In This Reaction.
From www.dreamstime.com
Catalyst Surface with Catalytic Reaction Stock Vector Illustration of Catalyst In This Reaction Catalysts play an important part in many chemical processes. catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. We have two different catalysts that speed up the. catalysts in biological reactions are called enzymes close enzyme a protein which catalyses or speeds up a chemical. catalyst, in. Catalyst In This Reaction.
From www.youtube.com
R2.2.6 Intermediates and catalysts (HL) YouTube Catalyst In This Reaction In heterogeneous catalysis, catalysts provide a surface to which reactants bind in a process of adsorption. what are catalysts, and how do they work in terms altering the parameters of a reaction? catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. We have two different catalysts that speed up the. Catalysts. Catalyst In This Reaction.
From www.labunlimited.com
Solid Phase Catalysis in Continuous Flow Chemistry Lab Unlimited Catalyst In This Reaction In heterogeneous catalysis, catalysts provide a surface to which reactants bind in a process of adsorption. Catalysts play an important part in many chemical processes. We have two different catalysts that speed up the. catalysts in biological reactions are called enzymes close enzyme a protein which catalyses or speeds up a chemical. a catalyst is a substance that. Catalyst In This Reaction.
From celimoko.blob.core.windows.net
Catalyst Reactions Examples at Jenny McNear blog Catalyst In This Reaction catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Catalysts play an important part in many chemical processes. catalysts in biological reactions are called enzymes close enzyme a protein which catalyses or speeds up a chemical. We have two different catalysts that speed up the. what are. Catalyst In This Reaction.
From www.expii.com
Catalysts (Enzymes) — Overview & Examples Expii Catalyst In This Reaction In heterogeneous catalysis, catalysts provide a surface to which reactants bind in a process of adsorption. Catalysts play an important part in many chemical processes. catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. catalysts in biological reactions are called enzymes close enzyme a protein which catalyses or. Catalyst In This Reaction.
From www.dreamstime.com
Enzyme As Catalyst in Chemical Reactions Stock Vector Illustration of Catalyst In This Reaction a catalyst is a substance that speeds up the rate of a chemical reaction. In heterogeneous catalysis, catalysts provide a surface to which reactants bind in a process of adsorption. what are catalysts, and how do they work in terms altering the parameters of a reaction? catalysts allow a reaction to proceed via a pathway that has. Catalyst In This Reaction.
From www.mometrix.com
What is a Catalyst? Chemistry Review (Video) Catalyst In This Reaction We have two different catalysts that speed up the. what are catalysts, and how do they work in terms altering the parameters of a reaction? a catalyst is a substance that speeds up the rate of a chemical reaction. catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed. Catalyst In This Reaction.
From www.youtube.com
A Catalyst and the Rate of Reaction YouTube Catalyst In This Reaction We have two different catalysts that speed up the. what are catalysts, and how do they work in terms altering the parameters of a reaction? catalysis is a process of increasing the rate of a chemical reaction by adding a chemical substance which is. a catalyst is a substance that speeds up the rate of a chemical. Catalyst In This Reaction.
From flatworldknowledge.lardbucket.org
Chemical Catalyst In This Reaction We have two different catalysts that speed up the. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. In heterogeneous catalysis, catalysts provide a surface to which reactants bind in a process. Catalyst In This Reaction.
From www.slideserve.com
PPT Mechanisms, Catalysts Intermediates and k PowerPoint Presentation Catalyst In This Reaction catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Catalysts play an important part in many chemical processes. catalysts in biological reactions are called enzymes close enzyme a protein which catalyses or speeds up a chemical. catalysts allow a reaction to proceed via a pathway that has a lower activation. Catalyst In This Reaction.
From www.slideserve.com
PPT Role of Catalysts PowerPoint Presentation, free download ID2823169 Catalyst In This Reaction In heterogeneous catalysis, catalysts provide a surface to which reactants bind in a process of adsorption. catalysts in biological reactions are called enzymes close enzyme a protein which catalyses or speeds up a chemical. what are catalysts, and how do they work in terms altering the parameters of a reaction? a catalyst is a substance that speeds. Catalyst In This Reaction.
From ar.inspiredpencil.com
Exothermic Reaction With Catalyst Catalyst In This Reaction catalysis is a process of increasing the rate of a chemical reaction by adding a chemical substance which is. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. We have two different catalysts that speed up the. a catalyst is a substance that speeds up the rate of a chemical. Catalyst In This Reaction.